Ph Value of Ethanoic Acid
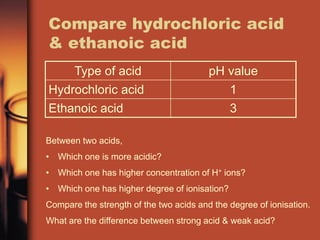
Now the acid is completely neutralized. Hydrochloric acid and sulphuric acid are examples of strong acid.
The Ph Of An Acetic Acid Solution Is 3 26 What Is The Concentration Of Acetic Acid And What Is The Percent Of Acid That S Ionized Quora
The concentration of the solution greatly affects the dissociation to form the hydrogen ion and the conjugate base acetate CH 3 COO At a concentration comparable to that in vinegar 10 M the pH is around 24 and only around 04 percent of the acetic acid molecules.
. Factors Determining Acid Strength. The thermometer shows the initial temperature reading which is room temperature. The lower values are obtained by adding sulfuric acid and higher values by adding solutions of ethanoic acid and ammonium sulfate or ammonium ethanoate.
It is produced by fermentation of carbohydrates or by organic synthesis. Hydrochloric acid is a strong acid - virtually 100 ionised. Further addition of such a small amount as 001 mL of the alkali raises the pH value by about 3 units to pH 7.
Try this set of experiments to compare the effects of light- and heavy-duty detergents with different pH values. Working out the pH of a strong acid. Ph Eur - Find MSDS or SDS a COA data sheets and more information.
An acid having a greater degree of dissociation. Dissociation constant of weak acid acetic acid can be determined by finding the value of degree of. Includes kit list and safety instructions.
Acetic acid concentration 000128 mol lit has an equivalent conductivity value of 004815 ohm-1 A. It occurs naturally in plant and animal tissues. Comparing the melting points of solder tin and lead.
The pH chosen for the solution in the dyebath depends on the individual properties of the dyes. Acetic acid glacial 100 CAS 64-19-7 anhydrous for analysis EMSURE ACSISOReag. All you have to do is work out the concentration of the hydrogen ions in the solution and then use your calculator to convert it to a pH.
Test the melting points of lead tin and solder to investigate solder as a solid mixture and alloy in this practical. With strong acids this is easy. In association with Nuffield Foundation.
Thus near the end point there is a rapid increase of pH from about 4 to 9. Sodium carbonate powder is tipped into a beaker of ethanoic acid. 64-19-7 is known as ethanoic acid.
Further of about 001 mL of 01 M NaOH will amount to adding hydrogen ions and the pH value will jump to about 9. Sodium sulfate may be added to control the diffusion of the dye anions in the fibre structure. This makes acetic acid a monoprotic acid with a pKa value of 476 in aqueous solution.
Ethanoic acid citric acid present in citric fruits and acetic acid present in vinegar are a few examples of weak acids. The principal synthetic methods currently employed are oxidation of acetaldehyde derived from ethylene liquid phase oxidation of butane and reaction of carbon. A Acetic acid C2H4O2 CAS Reg.
As already discussed different acids have different acid strengths. Suppose you had to work out the pH of 01 mol dm-3 hydrochloric acid.


No comments for "Ph Value of Ethanoic Acid"
Post a Comment